New Insights for Flow Imaging Microscopy Across Drug Product Development A recent “Stimuli to the Revision Process” article¹ from the US Pharmacopeia …
Read Post

Pharmaceutical researchers face mounting pressure to track and control particles in their drug formulations throughout development. Strategies to …
Read Post
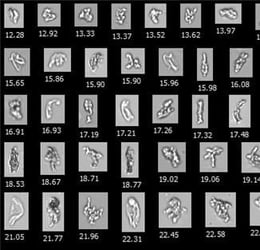
Light obscuration (LO) is the compendial method for quantifying subvisible particles (SVPs) in injectable drugs, harmonized across the US …
Read Post

A conversation with HTD Biosystems HTD Biosystems provides services to accelerate the development of biotech drugs, liposomal drug delivery systems, …
Read Post
The protein aggregate content in protein therapies and other biological drug products is often a critical quality attribute that must be monitored. …
Read Post

Protein aggregates in protein therapies can be difficult to monitor and measure in protein formulations due to their high transparency and irregular …
Read Post

Light obscuration (LO) is currently the compendial method for quantifying subvisible particles equal to or greater than 10 µm and 25 µm in biologic …
Read Post

Strategies for monitoring particles in biopharmaceutical formulations are essential in the development and manufacturing of safe, effective drug …
Read Post
Silicone oil droplets are a common particle type found in protein-based biotherapeutics, often seen in products packaged as prefilled syringes. …
Read Post
Particulates are ubiquitous in parenteral drug products and remain a concern throughout their development and production. These particles must be …
Read Post

Agitation during transportation is one of the most ubiquitous stress conditions to which therapeutic protein formulations and other biotherapeutic …
Read Post
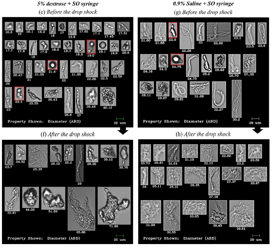
Protein aggregation is a major concern for biopharmaceuticals in post-production. Subvisible particles that form due to routine handling compromise …
Read Post






