Biopharma
FlowCam for AAV Aggregation Detection in Gene Therapies
Assess the stability and quality of the next generation of biotherapeutics designed to treat cancer, immunize against pathogens, and address genetic disorders.
Flow imaging microscopy detects aggregation of viral vectors such as AAVs, lentiviruses, and retroviruses as well as non-viral therapies like LNPs and exosomes.

Use FlowCam to:
- Monitor viral and nonviral vector aggregation in gene therapy formulations and drug products during formulation and process design
- Distinguish between inherent vector aggregates, intrinsic particles like glass flakes, and extrinsic contaminants
- Obtain reliable particle size and concentration measurements recommended by USP <1788> to support quality control processes
- Characterize particles as small as 300 nm with industry-leading image quality
Helpful Resources
“With FlowCam we get to see the particle morphology instead of just counts, and that really helps facilitate the debugging process if we encounter any issues during the development of new products.”
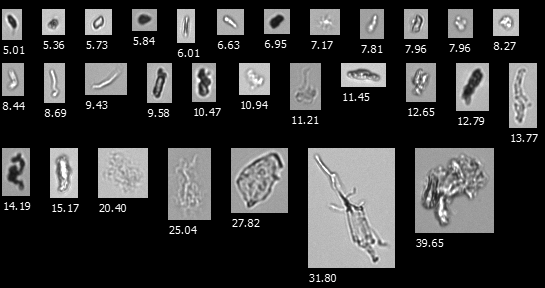

Sample FlowCam images of lentivirus aggregates

A FlowCam collage of particles in a heat-stressed LNP formulation
Formulation Development of Viral and Non-Viral Vectors
Like other parenteral biotherapeutics, injectables in gene therapy are subject to regulations like USP <788> that require particle testing to mitigate safety risks.
Flow imaging microscopy techniques allow for the identification and characterization of particle types in your formulation, enabling you to make formulation and process improvements during product development.
- Meet USP requirements and recommendations with FlowCam LO—a combined flow imaging and light obscuration instrument
- Detect gene vector aggregates such as LNP oligomers as small as 300 nm with FlowCam Nano
Additional Resources
 Videos
Videos
 White Papers
White Papers
- USP Reference Standards for Subvisible Particulate Matter
- Monitoring Subvisible Particles in Biotherapeutics
- Orthogonal and Complementary Techniques: Combining FIM with LO to Characterize Biotherapeutics
- Early Detection of Aggregation in Formulations: FlowCam Nano for submicron particle characterization in biotherapeutics

Interested in learning more?
-
Get in Touch
Tell us about your application and particle characterization needs.
-
Have a Conversation
We're happy to set up a call to discuss your application and answer your questions.
-
Discuss Next Steps
Expand your knowledge with a seminar, demonstration, sample analysis, or obtain a quote.