Biopharma
Advanced Subvisible Particle Analysis for Biotherapeutics Development
Biotherapeutics hold great promise, but their complexity makes them especially prone to particle formation. During processing, handling, and storage, these medicines can generate particles that conventional counting methods may overlook. If undetected, such particles can undermine safety, efficacy, and regulatory approval.
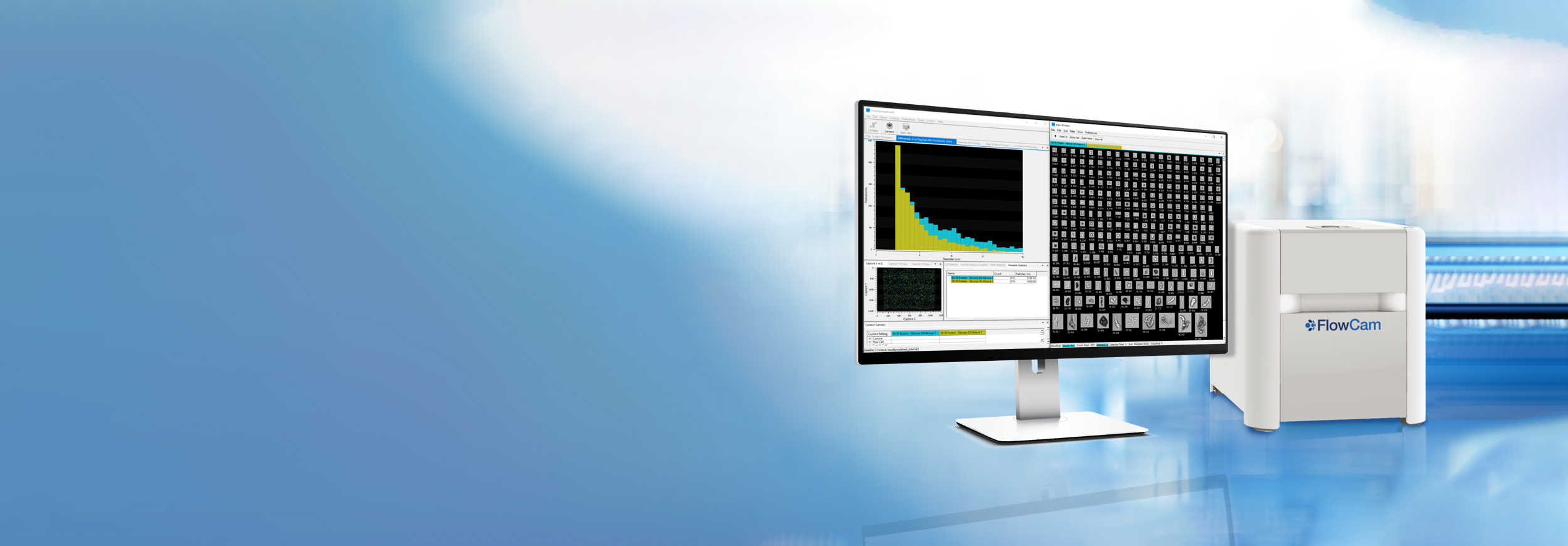
FlowCam delivers clarity in biotherapeutic particle analysis. Combining precise particle concentration measurements with high-resolution imaging, FlowCam reveals both how many particles are present and what they are. This enhanced visibility supports compliance with pharmacopeia guidelines and gives development teams the confidence to optimize formulations and protect patient safety.

Characterize Critical Particles Across All Modalities
Unlike small molecules, biologics are structurally delicate. Manufacturing and storage stresses affect stability, often causing aggregation and generating particles that can trigger immune responses or other adverse effects.
The challenge goes beyond detection—it’s precise characterization. Traditional light obscuration provides counts but lacks the visual detail needed for confident decisions. Without that context, formulation and manufacturing choices may depend on incomplete data, risking delays, failed batches, or worse: safety issues that derail years of development.
Helpful Resources
“There is great value in seeing images of the particles and of the actual contaminants. It allows us to identify particle type and particle source via morphology, which increases the strength of the finding.”

FlowCam images of particles in a biotherapeutic sample, including protein aggregates, silicone oil droplets, fibers, and other contaminants
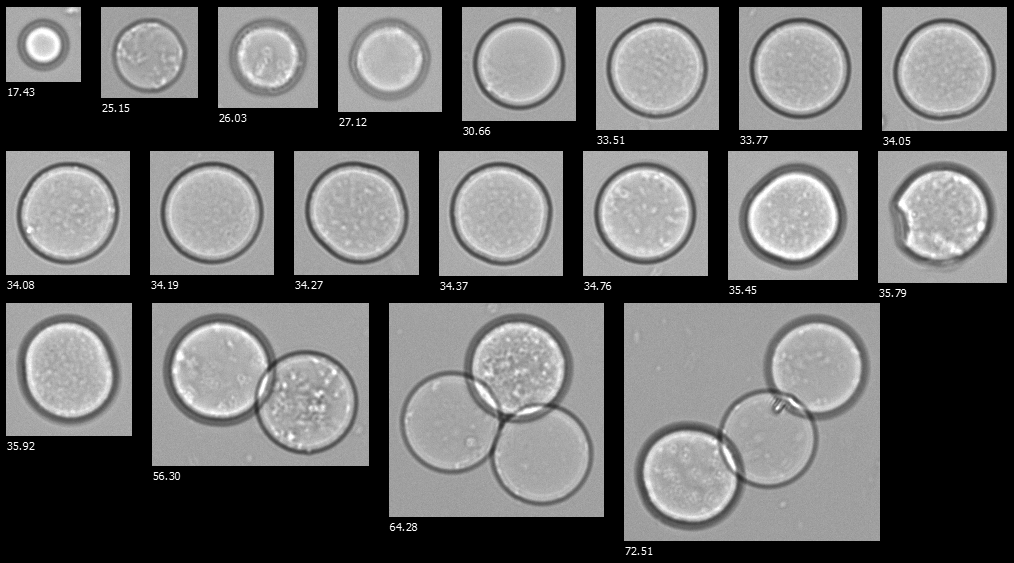
A FlowCam collage of hydrogel microspheres

A FlowCam collage of Dynabeads
How FlowCam Strengthens Biotherapeutic Development
FlowCam turns uncertainty into actionable insight by combining quantitative data with morphological confirmation in a single workflow. This dual approach helps biopharma teams see beyond counts to the particle types that truly impact product quality and safety.
With FlowCam, you can:
- Differentiate protein aggregates, silicone oil droplets, and other translucent particles undetectable by light obscuration
- Visualize particle formation to guide formulation development and prevent late-stage surprises
- Monitor particle profiles consistently across development, scale-up, and commercial manufacturing
- Generate comprehensive particle data aligned with USP <788> recommendations using FlowCam LO
- Comply with USP <1788> requirements with FlowCam 8000
- Rapidly identify whether failed batches result from degradation, contamination, or packaging issues
Critical Questions About Biotherapeutics Particle Analysis
-
Light obscuration and counting methods work well for opaque particles but struggle with translucent particles such as protein aggregates, which pose the greatest immunogenic risk to patients. These semi-transparent aggregates have refractive indices similar to aqueous formulations, making them nearly invisible to traditional detection methods. Particles not measured in solution but on a membrane substrate resolve these issues; however, they can induce changes in the number and types of particles detected.
FlowCam offers a solution-based imaging approach to measure translucent particles in their native buffer environment. These measurements reveal critical particles that counting methods miss entirely, giving you complete visibility into your particle profile.
-
FlowCam LO combines USP <788> compendial light obscuration data with imaging analysis in one efficient run. This satisfies regulatory requirements and accelerates investigations, enabling faster decisions, whether issues stem from API degradation, process contamination, or container problems, to keep manufacturing timelines on track.
-
Stability testing requires an understanding of particle behavior over time. FlowCam tracks morphological changes that indicate specific degradation pathways, providing the visual evidence that explains particle behavior in regulatory stability submissions. This data supports formulation modifications and enables accurate prediction of long-term product performance under different stress conditions.
-
The greatest impact occurs in the drug development phases, when particle specifications are most critical for regulatory viability. Early adoption also streamlines process development, stability profiling, and scale-up, ensuring fewer surprises during commercialization.
-
FlowCam distinguishes between protein aggregates, silicone oil droplets, glass particles, metallic fragments, rubber stopper material, and other process contaminants.
Each particle type carries unique morphological signatures that enable precise identification and corrective action, giving teams the complete particle profile they need to make informed formulation and quality decisions.
Additional Resources

Interested in learning more?
-
Get in Touch
Tell us about your application and particle characterization needs.
-
Have a Conversation
We're happy to set up a call to discuss your application and answer your questions.
-
Discuss Next Steps
Expand your knowledge with a seminar, demonstration, sample analysis, or obtain a quote.









