Agitation during transportation is one of the most ubiquitous stress conditions to which therapeutic protein formulations and other biotherapeutic drug products are exposed. These stresses often accelerate the formation of protein aggregates and other subvisible particles that pose product quality and safety risks. Measuring the particle content in protein drug products after accelerated or real-time shipping stresses can help researchers ensure that these therapies can be safely shipped to the patients that need them.
Several published studies have shown the impact of shaking on particle generation in monoclonal antibody (mAb) formulations. One recent study focuses on aggregation induced by pneumatic tube systems in hospitals. Relatively few of these studies have focused on monitoring the stability of protein formulations once reconstituted and/or diluted to their final concentration for use in infusion bags, a common form of transportation.
In “An Exploratory Study on the Effect of Mechanical Stress on Particle Formation in Monoclonal Antibody Infusions”, Mona Abdel-Tawab et al. investigated the impact of mechanical agitation on bevacizumab and 10 other mAb-based drug products when transported in infusion bags. The researchers used FlowCam 8100 along with dynamic light scattering (DLS) to compare particle concentrations present in these samples with and without shaking as well as at different protein concentrations and different time periods in storage.
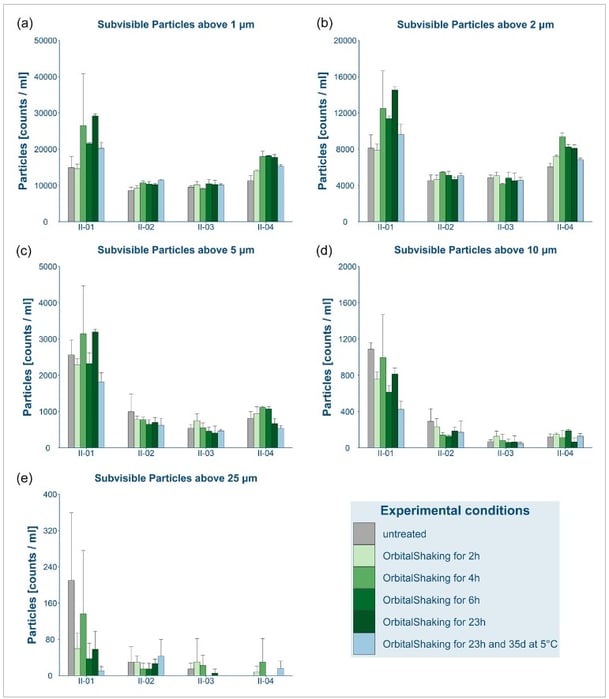 Their measurements indicated an increase in subvisible particle concentrations—particularly those smaller than 10 μm—following agitation. These agitation-based differences were especially noticeable at low concentrations of the drug product, potentially due to the subsequent lower concentrations of stabilizing excipients like surfactants. Samples that experienced extended storage times, unnecessary use of inline filtration, and/or the use of siliconized syringes also showed a higher concentration of subvisible particles.
Their measurements indicated an increase in subvisible particle concentrations—particularly those smaller than 10 μm—following agitation. These agitation-based differences were especially noticeable at low concentrations of the drug product, potentially due to the subsequent lower concentrations of stabilizing excipients like surfactants. Samples that experienced extended storage times, unnecessary use of inline filtration, and/or the use of siliconized syringes also showed a higher concentration of subvisible particles.
Pictured here: Abdel et al. Figure 5: "Overview of the total particle counts determined for bevacizumab at different dilutions (II-01 = 2, II-02 = 5, II-03 = 10, II-04 = 15 mg/mL) before and after orbital shaking at 600 rpm for up to 23 h at 2–8°C followed by storage at 2–8°C for 35 days."
The study’s findings highlight the adverse impact of mechanical stress and infusion bag mishandling on the particle content in mAb infusions at the point of patient administration. The authors offer practical advice for pharmacists and clinicians on handling these treatments to maximize the safety and efficacy of the drug products. They also recommend monitoring particle content smaller than 10 µm in mAb drug products and infusions—both during further “in use” stability studies and as part of routine quality control.
Read the full study:
Abdel-Tawab et al. An Exploratory Study on the Effect of Mechanical Stress on Particle Formation in Monoclonal Antibody Infusions. Archiv der Pharmazie. 2023;356(8). doi:10.1002/ardp.202300101











