Assessing subvisible particle content is a required quality control step for biotherapeutics and other parenteral drug products. The United States Pharmacopeia (USP) and in particular, USP <788> specifies that companies monitor the number of subvisible particles larger than 10 and 25 μm present in any commercialized parenteral drug product—including most protein, gene, and cell therapies. It also requires this testing to be done using either light obscuration (LO) or membrane microscopy, the former of which is the preferred technique. These requirements are harmonized with those in the European and Japanese pharmacopeias.
Light obscuration measurements have been shown to exhibit some limitations for analyzing common particles in biotherapeutic formulations. These measurements are relatively insensitive to translucent particles like protein and viral vector aggregates and will undersize and often undercount these particles. They also do not capture any information about the shape and source of the particles in the sample.
Flow imaging microscopy (FIM) is an orthogonal subvisible particle measurement technique that mitigates the issues of LO, offering greater sensitivity to translucent particles as well as information about particle type. USP recommends using FIM as an orthogonal technique to LO as part of routine quality control of biotherapeutics via USP <1788>.
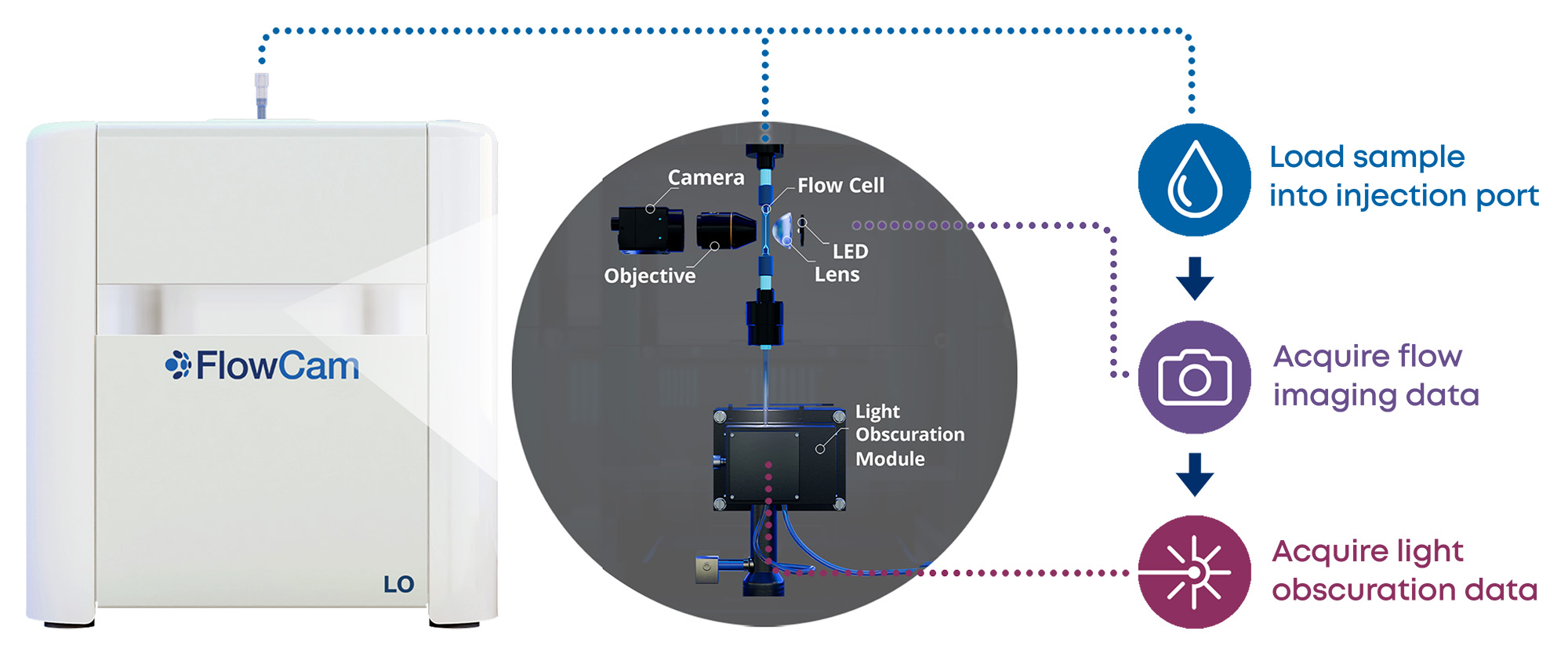
FlowCam LO instruments provide an innovative particle characterization solution by allowing researchers and technicians to capture both the required light obscuration and the recommended FIM subvisible particle data using a single instrument and single sample aliquot. In addition to capturing two orthogonal subvisible particle measurements, FlowCam LO provides light obscuration measurements that meet the requirements for USP <788> and <787> testing, as well as many of the recommendations for LO measurements found in USP <1787> and <1788>.
Pictured below: a schematic showing how flow imaging and LO data are captured with FlowCam LO
Learn how FlowCam LO meets both light obscuration requirements and flow imaging recommendations in our new Technical Notes:
Meeting USP standards for Subvisible Particulate Matter with FlowCam LO – Light Obscuration Method
 This document provides a thorough review of how the LO module in FlowCam LO meets the compendial requirements and recommended USP standards for the light obscuration method.
This document provides a thorough review of how the LO module in FlowCam LO meets the compendial requirements and recommended USP standards for the light obscuration method.
References are made to:
- USP <787> Particulate Matter in Therapeutic Protein Injections
- USP <788> Particulate Matter in Injections
- USP <789> Particulate Matter in Ophthalmic Solutions
- USP <1788> Methods for the Determination of Particulate Matter in Injections and Ophthalmic Solutions, specifically:
- <1788.1> Light Obscuration Method for the Determination of Subvisible Particulate Matter>
- <1788.1> Light Obscuration Method for the Determination of Subvisible Particulate Matter>
Meeting USP standards for Subvisible Particulate Matter with FlowCam – Flow Imaging Method
 This document describes how FlowCam 8100 and FlowCam LO meet USP recommendations for the flow imaging method.
This document describes how FlowCam 8100 and FlowCam LO meet USP recommendations for the flow imaging method.
References are made to:
-
USP <1788> Methods for the Determination of Particulate Matter in Injections and Ophthalmic Solutions, specifically:
-
USP <1788.3>: Flow Imaging Method for the Determination of Subvisible Particulate Matter
-