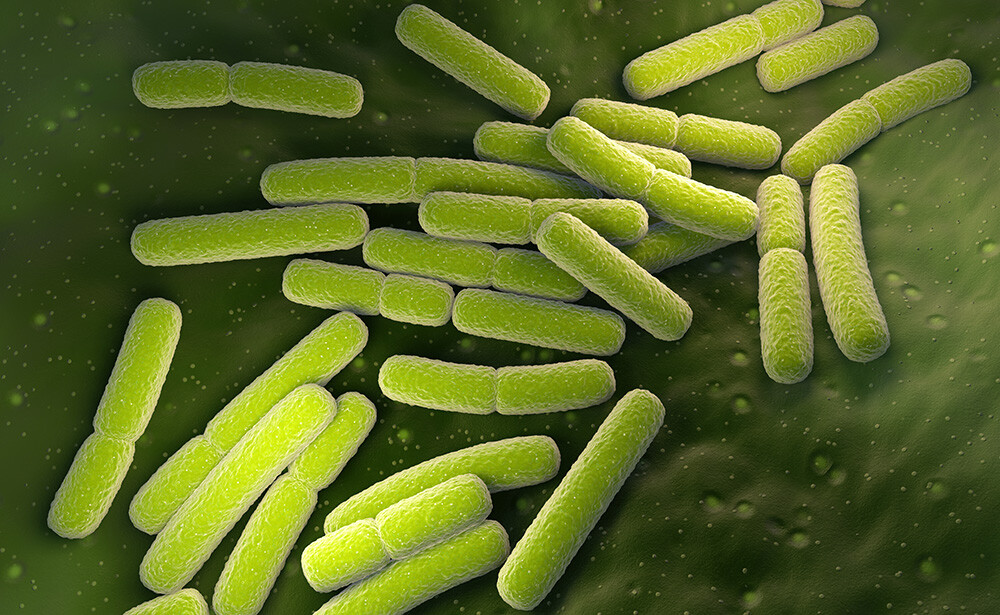
One of the main values of using flow imaging microscopy (FIM) when analyzing pharmaceuticals is the method's sensitivity to transparent particles. Many common particles in biopharmaceutical samples exhibit similar refractive indices to their suspension media, making them hard to see. FIM instruments tend to be more sensitive than other common analytical techniques like light obscuration (LO), allowing researchers to find unexpected particles in their samples that may not have been detected by other methods. Furthermore, the particle morphology information gleaned from FIM can help researchers identify what these surprise particles are and determine their source.
In a recent experiment, we used FlowCam LO, an instrument that performs simultaneous FIM and LO particle measurements, to analyze a suspension of PMMA microspheres. When we first analyzed the sample, the sample exclusively contained PMMA beads. However, when the sample was analyzed again a few days later, a large population of small (2-4 μm) rod-like particles were observed in the FIM images but not observed in the size distribution reported by LO. The measured particle size distribution and sample images of these particles are shown below.
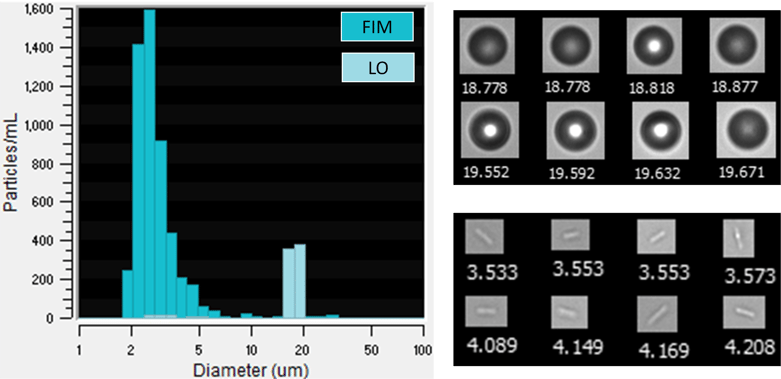
(Left) Particle size distributions as measured by FIM and LO. LO data is overlain on the FIM data, masking the peak in the FIM data near 19 μm. (Right, top) Sample FlowCam FIM images of PMMA spheres. (Right, bottom) Sample images of rod-like particles that are potentially bacteria cells. Collages are not to scale relative to each other. Values below each image in the collages are Diameter (ESD) values in μm.
While we were unable to ultimately identify what these particles were, their size and shape, in addition to the timing of their appearance, likely indicate that they are bacteria cells. Bacterial contamination is a severe quality issue for any drug product as it can adversely impact product safety. For example, in early 2022 bacterial contamination of eye drops and other ophthalmic products was associated with infections of a multi-drug resistant strain of Pseudomonas aeruginosa in patients.
As our PMMA suspension data shows, FIM instruments can help detect potential bacterial contamination in biologic drugs. FlowCam Nano is often the preferred FIM instrument for bacteria monitoring due to its sensitivity to particles 0.5-2 μm in size—the size range of most bacteria. However, typical FlowCam 8000 series instruments, including FlowCam LO, can detect some larger bacteria particles to help researchers identify possible contamination issues and prompt them to investigate further.
Many subvisible particle analysis techniques commonly used for biopharmaceutical quality control testing may not be able to detect bacterial contamination if used alone. This likely stems from a combination of the size and transparency of bacteria. For example, bacteria are near the lower size detection limit of LO and, due to the sensitivity of the technique to particle transparency, bacteria may falsely be assigned sizes below the lower size limit of the measurement. While the lower size range of FIM is comparable to that of LO, the higher robustness of FIM to particle transparency allows small bacterial cells to be detected, sized correctly, and counted.
FlowCam can be useful in identifying samples where additional microbiology testing may be warranted, especially relative to other subvisible particle analysis methods. While FIM alone does not replace standard microbial testing methods, bacteria are one of many relevant particle types that FlowCam can analyze—including protein and viral vector aggregates as well as viable and nonviable cells. This makes instruments like FlowCam LO valuable in identifying the sources of particles in a biotherapeutic sample and getting unwanted particle populations under control.
For FlowCam users interested in identifying bacteria in their samples, here are some technical tips you might find helpful:
- Use instrument settings similar to those used for standard biopharmaceutical samples.
- Pay close attention to particles between 2-5 μm in diameter.
- When present, bacillus-shaped bacteria species such as E. coli often result in elongated, low aspect ratio particles like those shown above.
- Staphylococcus and streptococcus morphologies are likely harder to detect at 10X resolution but may also be observed.
Learn more: