Subvisible particles in biotherapeutics represent critical quality attributes because they pose regulatory and potential product safety risks. Therefore, much of the development work behind these therapeutics focuses on creating formulations and manufacturing processes that minimize the particle content in the drug product and reduce the potential for adverse immunogenicity.

Pictured above are images of IV bags before and after transport via Pneumatic Tube System with Dextrose and Saline.
Despite measures taken during formulation and manufacturing, product quality can still be compromised after manufacturing due to accidental stresses caused by freeze-thawing, exposure to light, and mechanical shock from dropping the drug container. In a recent collaboration between the University of Colorado and Children's Hospital Colorado, researchers investigated particle generation in IV bags containing therapeutic protein formulations in the hospital's pneumatic tube system (PTS). Particle monitoring technologies like FlowCam are critical to ensure biotherapeutics have acceptable particle content not just after manufacturing but up until the therapy is administered to patients.
While many hospitals forbid the transport of therapeutic protein formulations via pneumatic tube systems, it remains a problem in some settings. In this study, Flow Imaging Microscopy (FIM) via FlowCam and Light Obscuration (LO) via HIAC were used to characterize the particle content of IV bags before and after transport via PTS. The findings indicated that IV bags contained significantly higher particle concentrations after being sent via PTS with the exact increase varying by IV bag materials (polyvinyl chloride or polyolefin), the protein formulation (IVIG or mAb), and the buffer solution (Dextrose or saline).


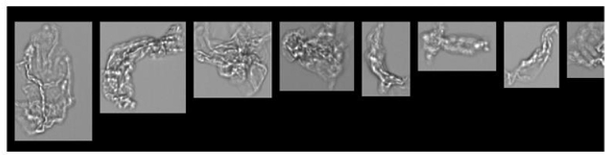
FlowCam images of particles formed in PVC IV bags filled with saline and no protein (top), mAb (middle), or IVIG (bottom) following PTS transport. The circular particles in the top two rows are likely plasticizer droplets. The amorphous particles in the bottom two rows are likely protein aggregates.
FlowCam images following PTS transport suggest that these new particles consist not only of protein aggregates but also plasticizer droplets which have been known to promote further protein aggregation. Light obscuration measurements performed on these samples were consistent with the lower sensitivity of this modality to biotherapeutic-relevant particles and did not detect increased particle concentration following transport.
Read the full publication here: Effects of Transportation of IV Bags Containing Protein Formulations Via Hospital Pneumatic Tube System: Particle Characterization by Multiple Methods: Linkuviene, Ross, Crawford, Journal of Pharmaceutical Sciences January 2022.
This study demonstrates that sending therapeutic protein formulations via PTS can result in significant particle generation, greatly reducing the quality of the therapeutic that a patient ultimately receives. The authors strongly advise against using PTS to move these therapies in hospitals. Characterizing these downstream particle sources with FlowCam can help researchers develop more effective guidance for handling these therapies, minimize particle formation after a sample is manufactured, and ensure that patients receives the highest quality therapeutic possible.