New Insights for Flow Imaging Microscopy Across Drug Product Development
A recent “Stimuli to the Revision Process” article¹ from the US Pharmacopeia (USP) explains the rationale for adding an informational chapter to the USP Guidelines focused specifically on subvisible silicone oil particles (SiOPs). The article highlights how this chapter addresses ongoing challenges in characterizing subvisible particles (SbVPs) in protein therapies per USP <787>, in parenteral drug products per USP <788>, and more broadly in particulate matter for therapeutic injections and ophthalmic solutions per USP <1788>.
As prefilled syringes lubricated with silicone oil have become the preferred primary container for biologics, it is now necessary to introduce orthogonal analytical techniques for characterizing subvisible particles. The article suggests applying orthogonal methods to accurately detect, quantify, and characterize silicone oil particles, thereby establishing a comprehensive understanding of drug products and helping manufacturers define mitigation strategies for products exceeding compendial limits. The Stimuli article emphasized the importance of distinguishing silicone oil from other subvisible particles using flow imaging microscopy (FIM).
FlowCam Differentiates Between Silicone Oil and Other Subvisible Particles
High-resolution images obtained with FlowCam, a flow imaging microscope, enable clear differentiation of particle type. FlowCam can detect and categorize particles >2 µm by applying morphology-based classification to distinguish spherical SiOPs from amorphous protein aggregates or other inherent, intrinsic, and extrinsic particulates. This differentiation is not possible with the limited information provided by LO or membrane microscopy. FIM provides a better understanding of particle sources in addition to quantitative data that can be used to track particle content throughout the drug development lifecycle.
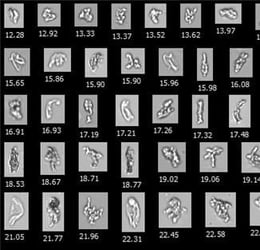
![]() Figure 1. Compared to light obscuration (LO), FIM detects a greater number of particles in the 2–10 µm range, and provides images and morphological data to easily distinguish SiOPs from protein aggregates.
Figure 1. Compared to light obscuration (LO), FIM detects a greater number of particles in the 2–10 µm range, and provides images and morphological data to easily distinguish SiOPs from protein aggregates.
Why Silicone Oil Particles Matter
Silicone oil particles are an important consideration in protein-based therapeutics, especially when products are delivered in prefilled syringes. Because silicone oil is used as a lubricant in the container–closure system, droplets can migrate into the formulation and vary in concentration depending on how the syringe is siliconized or handled during manufacturing and storage. While the immunogenicity of SiOPs is not yet fully understood, the abundance of these particles in drug products warrants concern due to their tendency to form particle–protein complexes that could stimulate immune responses.
These subvisible droplets are challenging to detect with conventional particle analysis techniques. In contrast, FIM provides the visual detail needed to distinguish them from protein aggregates and other particulates. Monitoring silicone oil droplets not only helps identify potential issues with the container–closure system but also supports a deeper understanding of how these droplets may interact with therapeutic proteins, potentially influencing stability, aggregation behavior, and overall product quality.
Interested in learning more about this topic? Make sure to register for the upcoming USP webinar:
Addressing Subvisible Silicone Oil Droplets—Industry Challenges, Analytical Strategies, and USP’s Rationale for a New General Informational Chapter
Time & Date:
Wednesday, March 4th, 10:00 AM EST
Speakers:
Desmond G. Hunt, Senior Principal Scientist, USP
Laura A. Philips, PhD, MBA, President and CEO, Spheryx, Inc.
Tyler Carter, PhD, Senior Application Scientist, Yokogawa Fluid Imaging Technologies
References
- USP Stimuli Article: Addressing Subvisible Silicone Oil Droplets – Industry Challenges, Analytical Strategies, and USP’s Rationale for a New General Informational Chapter