
Cell & Gene Therapy
AAV Aggregation Monitoring & Cell Therapy Quality Control
Assess the stability and quality of the next generation of biotherapeutics designed to treat cancer, immunize against pathogens, and address genetic disorders.
Flow imaging microscopy detects aggregation in formulations of viral vectors such as AAVs, lentiviruses, and retroviruses as well as non-viral therapies like LNPs or other gene vector carriers.
For CAR-T cells and other types of cell-based medicinal products, flow imaging microscopy monitors concentrations of viable and nonviable cells as well as particulate impurities.

Use FlowCam to:
- Monitor cell viability, gene vector aggregation, and product contamination
- Obtain particle type and morphology information to complement orthogonal sizing and counting techniques
- Characterize particles as small as 300 nm with industry-leading image quality
Helpful Resources
“In the last 5 years, the [cell and gene therapy] field has expanded dramatically, with unprecedented approvals. There is now a greater need to move the discussion toward particulate management in cell- and gene-specific regulatory guidance.”


A FlowCam collage of particles in a heat-stressed LNP formulation
Formulation Development of Viral and Non-Viral Vectors
Like other parenteral biotherapeutics, injectables in gene therapy are subject to regulations like USP <788> that require particle testing to mitigate safety risks.
Flow imaging microscopy techniques allow for the identification and characterization of particle types in your formulation, enabling you to make formulation and process improvements during product development.
- Meet USP requirements and recommendations with FlowCam LO—a combined flow imaging and light obscuration instrument
- Detect gene vector aggregates such as LNP oligomers as small as 300 nm with FlowCam Nano
CAR-T Therapy Applications
The inherently large size of therapeutic cells (4-15 µm) restricts the use of sterile filtration and other size-based separations to remove subvisible process impurities and contaminants like DynabeadsTM and cellular debris.
Flow imaging microscopy enables you to mitigate potentially harmful particles in your formulation and monitor cell viability.
- Differentiate between cells, cell-based impurities, and process-based impurities like Dynabeads to optimize product manufacturing
- Utilize image analysis tools in VisualSpreadsheet to measure cell concentrations and viability in samples without the need for fluorescence labeling
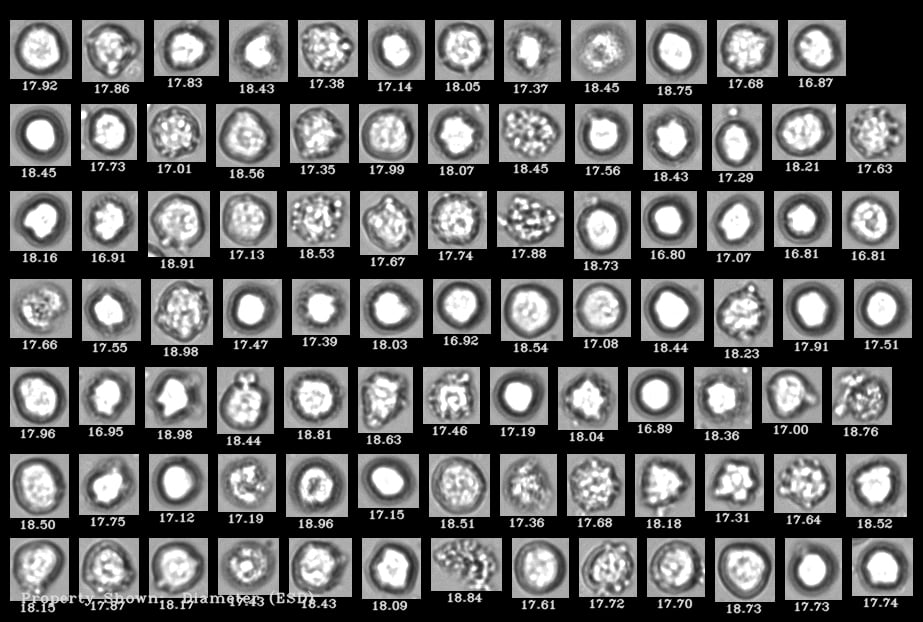
A FlowCam collage of human cells where living cells are distinguishable from dead cells
Additional Resources
 Application Notes
Application Notes
 White Papers
White Papers
 Published Research
Published Research
- Of Particulate Importance – Flow Imaging Microscopy in Cell and Gene Therapy
- Flow Imaging Microscopy Coupled with Convolutional Neural Networks Demonstrates Reliable Method to Characterize and Identify Impurities in Cell-Based Medicinal Products
- Flow Imaging Microscopy Guides Forced Degradation of Cell-based Medicinal Products

Interested in learning more?
-
Get in Touch
Tell us about your application and particle characterization needs.
-
Have a Conversation
We're happy to set up a call to discuss your application and answer your questions.
-
Discuss Next Steps
Expand your knowledge with a seminar, demonstration, sample analysis, or obtain a quote.









