Today’s drug manufacturers are under increasing pressure to understand the particulate composition of their drugs, and to reduce and control particulate in their formulations. To reduce and control particulate matter, manufacturers must first understand its source. Traditionally, particle analysis has been accomplished via light obscuration (LO) and membrane microscopy. While having been the accepted analytical standard methods for particulate composition as outlined by USP <788>, experts have deemed them to be insufficient.
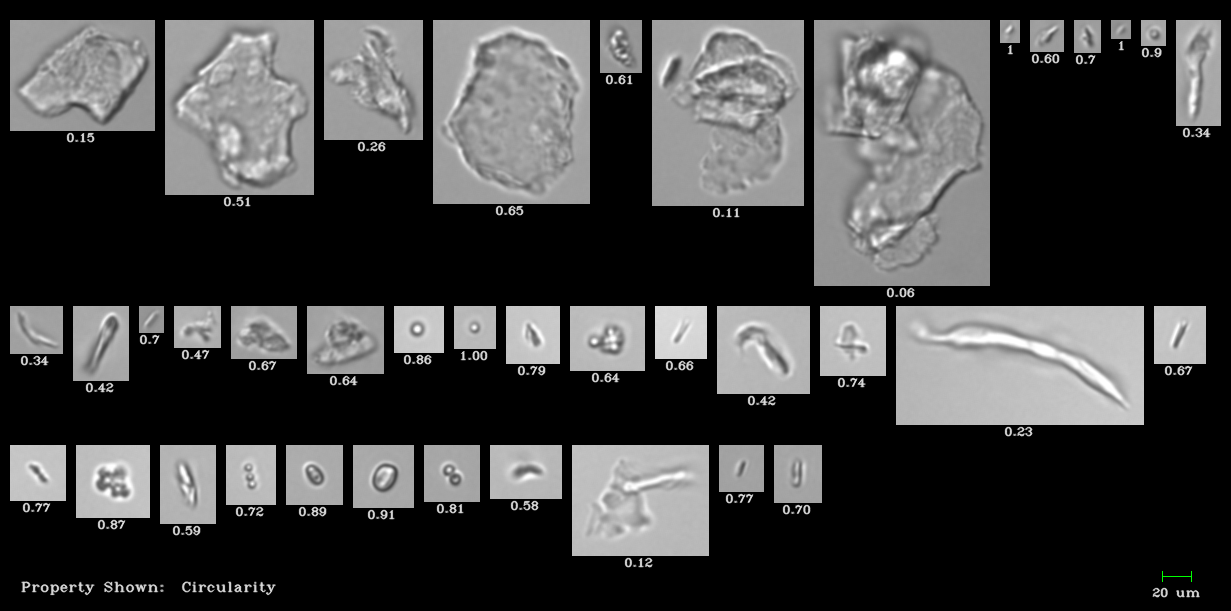
Pictured above: National Institute of Standards and Technology protein standard imaged by the FlowCam. Proteins and non-proteinaceous particles were captured by the FlowCam.
Particle images from flow imaging microscopes enable thorough particle characterization, higher quality assurance and ensure greater product safety. Flow imaging microscopy captures an image of each particle in the sample. These images are recorded and used to calculate particle measurements, such as size and concentration. Particle morphology can be used to identify the particle and its source, as well as observe any changes in protein nature that could make the therapeutic formulation ineffective or harmful.
Read more about the value of flow imaging microscopy in the latest issue of Laboratory Equipment.